HIV stands for Human Immunodeficiency Virus. It is a virus that assaults the immune system of the human body.
According to experts, untreated HIV can progress to AIDS (Acquired Immunodeficiency Syndrome).
Studies revealed that the virus can be transferred by unprotected intercourse (vaginal or anal) with an infected person, improperly regulated blood transfusion, mother-to-child transmission during pregnancy, childbirth, nursing, and sharing needles or syringes.
Early signs include fever, exhaustion, rash, sore throat, and weight loss, though many people may go asymptomatic for years.
According to recent estimates, around 43,683 persons in Nigeria would die from HIV-related causes by 2025. Of those, 28,589 were adults (13,650 males and 14,939 women), while 15,094 were children aged 0 to 14.
Over the years, medical authorities have highlighted various precautions to avoid contracting the human immunodeficiency virus (HIV). These precautions include the use of condoms, regular medical tests and avoiding sharing needles with others.
What is Lenacapavir?
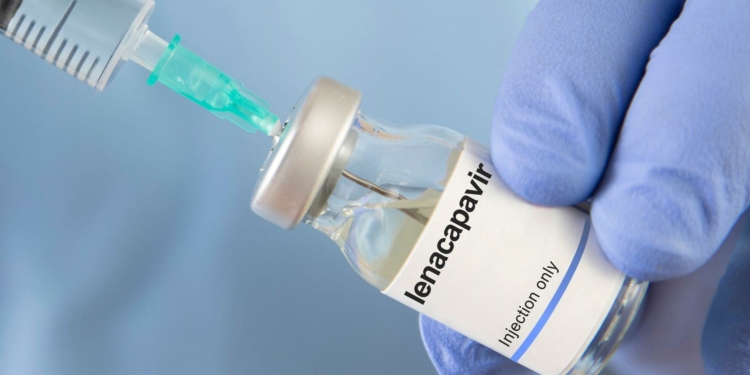
Lenacapavir is the first-in-class HIV-1 capsid inhibitor. It prevents the infection from spreading further. It inhibits the capsid protein, impairing the virus’s ability to replicate. It stops HIV from transferring its genetic material to host cells. It can also aid with resistance to other HIV medications.
In 2025, Nigeria will join world leaders in reporting a historic price drop for a new HIV preventive medicine. Under the agreement, the cost of lenacapavir, a twice-yearly injectable that has been shown to be up to 100% effective in preventing HIV infection, will be reduced from $28,000 to $40 per person per year.
A publication concerning Lenacapavir, a medicine used for HIV prophylaxis and treatment, said that it adheres to the viral capsid (p24) and “hyper-stabilizes” it. It prevents three critical steps: the nuclear import of viral DNA, the assembly of new particles, and core formation.
Lenacapavir targets a component of HIV that no other medicine does, and it remains effective against multi-drug-resistant strains. It is used to treat experienced adults with multidrug-resistant HIV in combination with other antiretrovirals. It is also used for HIV-negative adults and adolescents over 35 kg who are at high risk, reducing the possibility of sexually transmitted HIV.
Usage:
Lenacapavir can be administered as oral pills or as an injectable. When a shot is missed, oral tablets (300 mg) can be used for loading and bridging, while a subcutaneous injection (927 mg/1.5 mL) should be delivered every 6 months.
Day 1: 2 × 300 mg tablets + 2 × 1.5 mL injections (total 927 mg)
Day 2: 2 × 300 mg tablets
Maintenance: 2 × 1.5 mL injections every 26 weeks – (An alternative schedule starts with oral tablets on days 1, 2, 8, then injection on day 15)
When injections are given, patients or recipients may experience discomfort, swelling, nodules, itching, hardness, and moderate nausea. Serious adverse events may occur, but they are uncommon.
Acceptance?
Lenacapavir medication achieved a positive CHMP opinion, a regulatory test in the EU (June 2022), and marketing authorization in August 2022.
In November 2022, it was approved by a Canadian regulatory agency. The Food and Medicine Administration (FDA) authorized the medicine for treatment in December 2022 in the US, prioritizing review, fast-track, and breakthrough designations, among others.
Zimbabwe started a countrywide program that provides twice-yearly injections to approximately 46,000 high-risk patients, attempting to address daily pill adherence difficulties.
Lenacapavir’s unique mechanism gives it potency against resistant HIV and a long‑acting dosing schedule.
It’s now approved for both treatment and prevention in major markets, with strong trial data supporting its use.



Discussion about this post